- #1
nicky670
- 21
- 1
Homework Statement:: The relative density of water is determined by the rate at which it expands (and contracts) with changes in temperature. At approximately what other temperature T does water have the same density as at 1 ∘C ?
Relevant Equations:: Just looking at graphical and analyzing. But i can't seem to figure out why the answer is 8 degree celsius.
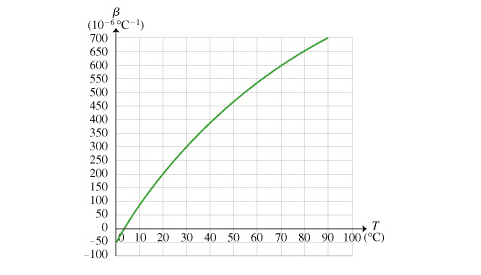

Relevant Equations:: Just looking at graphical and analyzing. But i can't seem to figure out why the answer is 8 degree celsius.