- #1
youmei0426
- 18
- 0
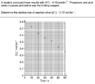
Homework Statement
Homework Equations
The Attempt at a Solution
I need to know the time it takes for the [H+] concentration to be 0.15mol/L. However, the graph gives me the [I2] concentration over time, and I don't know how to convert it to the [H+] concentration. How should I solve the problem? Thanks!