- #1
GravityX
- 19
- 1
- Homework Statement
- At what temperature does the degrees of freedom freeze (estimate)
- Relevant Equations
- none
Hi,
I am unfortunately stuck with the following task
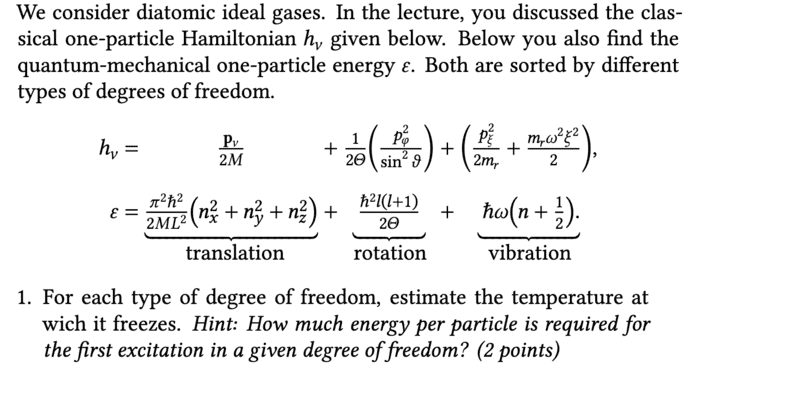
I started once with the hint that at very low temperatures the diatomic ideal gas behaves like monatomic gas and has only three degrees of freedom of translation ##f=3##. If you then excite the gas by increasing the temperature, you add two degrees of freedom of rotation, ##f=5## and if you then excite the gas even further, you add two more degrees of freedom of vibration ##f=7##.
The Equipartition theorem states that the internal energy is distributed equally among the degrees of freedom. The calculation of the internal energy for the ideal gas is ##U=\frac{3}{2}RT## for the translation, ##U=\frac{5}{2}RT## for the rotation and ##U=\frac{7}{2}RT## for the oscillation.
Unfortunately, I don't know either ##U## or ##T##, but I can't think of any other way to estimate the temperature.
I am unfortunately stuck with the following task
I started once with the hint that at very low temperatures the diatomic ideal gas behaves like monatomic gas and has only three degrees of freedom of translation ##f=3##. If you then excite the gas by increasing the temperature, you add two degrees of freedom of rotation, ##f=5## and if you then excite the gas even further, you add two more degrees of freedom of vibration ##f=7##.
The Equipartition theorem states that the internal energy is distributed equally among the degrees of freedom. The calculation of the internal energy for the ideal gas is ##U=\frac{3}{2}RT## for the translation, ##U=\frac{5}{2}RT## for the rotation and ##U=\frac{7}{2}RT## for the oscillation.
Unfortunately, I don't know either ##U## or ##T##, but I can't think of any other way to estimate the temperature.