- #1
shiznites
- 2
- 0
ORGANIC CHEMISTRY II
I spent hours trying to figure out how to do this mechanism but I always get stuck. Its tricky and will make you think.. think you can figure it out?
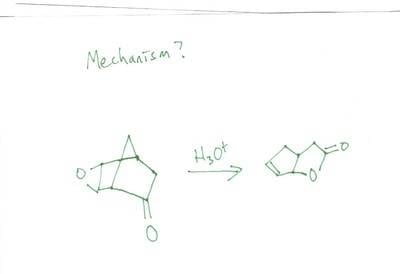
Figure out the mechanism just using acid (H+)
I started out by reducing the epoxide by protinating and was left with a -OH group on each carbon that made up the epoxide. Using one of those -OH groups I attack the carbonyl on the opposite side (using the -OH group as a nucleophile).
Basically I don't know where to go from there or if that's even a correct start. I sat here for awhile staring at my 3D model and came up nowhere. I know that somewhere down the line there will be dehydration somewhere.
Your help is greatly appreciated =]
Homework Statement
I spent hours trying to figure out how to do this mechanism but I always get stuck. Its tricky and will make you think.. think you can figure it out?
Homework Equations
Figure out the mechanism just using acid (H+)
The Attempt at a Solution
I started out by reducing the epoxide by protinating and was left with a -OH group on each carbon that made up the epoxide. Using one of those -OH groups I attack the carbonyl on the opposite side (using the -OH group as a nucleophile).
Basically I don't know where to go from there or if that's even a correct start. I sat here for awhile staring at my 3D model and came up nowhere. I know that somewhere down the line there will be dehydration somewhere.
Your help is greatly appreciated =]
Last edited: