- #1
mrandersdk
- 246
- 1
Hello
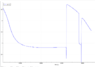
I have a problem when I dissolves NaCl in deionized water. I have a pH probe in my deionized water (lets say 1 L). Then I add 40 g of NaCl, imidiately the pH-value rises to about 9.0 (i'm logging hte pH value as a function of time). Then slowly the pH-value lowers, and after 11 hours !, it has stabalized at 6.8.
The probe is calibrated, and talking with the manufacture they confirm that we are using the instrument correctly. When we transfer the probe to a calibration solution it reacts instantly to the correct value, and when we transfer it back to the NaCl solution it, goes back to the pH value we have reached for the solution at that time (we don't go back to the 9.0, but enter at for example 7.8 if that where we are in the process, and then it continues down to the 6.8).
Anybody has an idea why the pH value would rise when I add the NaCl?
Anybody has an idea why the pH value takes so long to stabalize? Does it really take so long for NaCl to dissolve?
Any ideas how we could get it to stabalize faster?
I have a problem when I dissolves NaCl in deionized water. I have a pH probe in my deionized water (lets say 1 L). Then I add 40 g of NaCl, imidiately the pH-value rises to about 9.0 (i'm logging hte pH value as a function of time). Then slowly the pH-value lowers, and after 11 hours !, it has stabalized at 6.8.
The probe is calibrated, and talking with the manufacture they confirm that we are using the instrument correctly. When we transfer the probe to a calibration solution it reacts instantly to the correct value, and when we transfer it back to the NaCl solution it, goes back to the pH value we have reached for the solution at that time (we don't go back to the 9.0, but enter at for example 7.8 if that where we are in the process, and then it continues down to the 6.8).
Anybody has an idea why the pH value would rise when I add the NaCl?
Anybody has an idea why the pH value takes so long to stabalize? Does it really take so long for NaCl to dissolve?
Any ideas how we could get it to stabalize faster?