- #1
old_codger
- 8
- 0
hello PF community
i'm looking for help in understanding the classification and observed behaviour of some DIY voltaic (?) cells and their associated circuits with which I've been tinkering for a couple of years
the cell operation seems to belong somewhere between galvanic and the original 'contact tension' school of thinking because there appears to be no 'salt-bridge separating two half-cells' type of mechanism involved
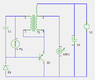
i've used flat foil electrodes (approx 3x3"; zinc anode; copper cathode) with thin cotton or paper separator, impregnated only with a little (1cc?) starch-based glue-stick or honey
the open-circuit voltage, per cell, was in the range 0.7V-0.9V with a very high internal impedance (only able to support 1uA load or less)
i've been loading these cells (or batteries of two) using very low current drain circuits, continously flashing a single LED at 0.5Hz or less
the cells (along with their load circuits) have been contained within either mild-steel or aluminium cases
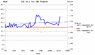
the first such DIY cell tested has been operating on-load since early 2011, the trend of the cell potential has continued to increase over the last 18 months approx.
comparison tests using commercial primary and secondary cells (Alkaline and NiMH AAA type) with similar load circuits have shown the trends of each cell potential to exhibit a slight but steady decrease of cell potential throughout their several-month test periods
firstly, is it possible to identify these DIY cells as being a particular type?
secondly, since there is no 'salt-bridge' type component, what 'mechanism' is enabling these cells to operate without some form of polarization starting to decrease the cell potential?
thanks in advance for any enlightenment!
i'm looking for help in understanding the classification and observed behaviour of some DIY voltaic (?) cells and their associated circuits with which I've been tinkering for a couple of years
the cell operation seems to belong somewhere between galvanic and the original 'contact tension' school of thinking because there appears to be no 'salt-bridge separating two half-cells' type of mechanism involved
i've used flat foil electrodes (approx 3x3"; zinc anode; copper cathode) with thin cotton or paper separator, impregnated only with a little (1cc?) starch-based glue-stick or honey
the open-circuit voltage, per cell, was in the range 0.7V-0.9V with a very high internal impedance (only able to support 1uA load or less)
i've been loading these cells (or batteries of two) using very low current drain circuits, continously flashing a single LED at 0.5Hz or less
the cells (along with their load circuits) have been contained within either mild-steel or aluminium cases
the first such DIY cell tested has been operating on-load since early 2011, the trend of the cell potential has continued to increase over the last 18 months approx.
comparison tests using commercial primary and secondary cells (Alkaline and NiMH AAA type) with similar load circuits have shown the trends of each cell potential to exhibit a slight but steady decrease of cell potential throughout their several-month test periods
firstly, is it possible to identify these DIY cells as being a particular type?
secondly, since there is no 'salt-bridge' type component, what 'mechanism' is enabling these cells to operate without some form of polarization starting to decrease the cell potential?
thanks in advance for any enlightenment!