- #1
kenshi64
- 34
- 0
1. Research Question: How effectively can experimental values estimate the heat of hydration of Copper (II) Sulfate as compared to theoretical values found using Hess’s law?
2.
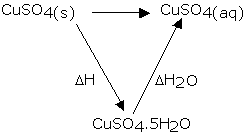
3. In the image above I have ΔH20 and ΔHreaction(not labelled) and intend to experimentally find out ΔH.
Problem: is that my textbooks states that equation can be used for Hess's Law if they're balanced. Are the equations in the image balanced? IMHO they're incomplete. That's my first problem, How can (aq) and (.5H20) be balanced?I prepared 0.025 solutions of both hydrated and anhydrous CuSO4 and measure their temperatures during hydration and forming of an aqueous solution respectively. IS that right?
Thank you for your interest! :D
2.
3. In the image above I have ΔH20 and ΔHreaction(not labelled) and intend to experimentally find out ΔH.
Problem: is that my textbooks states that equation can be used for Hess's Law if they're balanced. Are the equations in the image balanced? IMHO they're incomplete. That's my first problem, How can (aq) and (.5H20) be balanced?I prepared 0.025 solutions of both hydrated and anhydrous CuSO4 and measure their temperatures during hydration and forming of an aqueous solution respectively. IS that right?
Thank you for your interest! :D