Do Photons Move Slower in a Solid Medium?
This question appears often because it has been shown that in a normal, dispersive solid such as glass, the speed of light is slower than it is in a vacuum. This FAQ will strictly deal with that scenario only and will not address light transport in anomalous medium, atomic vapor, metals, etc., and will only consider light within the visible range.
The process of describing light transport via the quantum mechanical description isn’t trivial. The use of photons to explain such a process involves the understanding of not just the properties of photons, but also the quantum mechanical properties of the material itself (something one learns in Solid State Physics). So this explanation will attempt to only provide a very general and rough idea of the process.
A common explanation that has been provided is that a photon moving through the material still moves at the speed of c, but when it encounters the atom of the material, it is absorbed by the atom via an atomic transition. After a very slight delay, a photon is then re-emitted. This explanation is incorrect and inconsistent with empirical observations. If this is what actually occurs, then the absorption spectrum will be discrete because atoms have only discrete energy states. Yet, in glass, for example, we see almost the whole visible spectrum being transmitted with no discrete disruption in the measured speed. In fact, the index of refraction (which reflects the speed of light through that medium) varies continuously, rather than abruptly, with the frequency of light.
Secondly, if that assertion is true, then the index of refraction would ONLY depend on the type of atom in the material, and nothing else, since the atom, is responsible for the absorption of the photon. Again, if this is true, then we see a problem when we apply this to carbon, let’s say. The index of refraction of graphite and diamond are different from each other. Yet, both are made up of carbon atoms. In fact, if we look at graphite alone, the index of refraction is different along with different crystal directions. Obviously, materials with identical atoms can have a different index of refraction. So it points to the evidence that it may have nothing to do with an “atomic transition”.
When atoms and molecules form a solid, they start to lose most of their individual identity and form a “collective behavior” with other atoms. It is as the result of this collective behavior that one obtains a metal, insulator, semiconductor, etc. Almost all of the properties of solids that we are familiar with are the results of the collective properties of the solid as a whole, not the properties of the individual atoms. The same applies to how a photon moves through a solid.
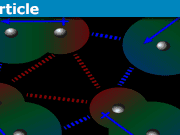
A solid has a network of ions and electrons fixed in a “lattice”. Think of this as a network of balls connected to each other by springs. Because of this, they have what is known as “collective vibrational modes”, often called phonons. These are quanta of lattice vibrations, similar to photons being the quanta of EM radiation. It is these vibrational modes that can absorb a photon. So when a photon encounters a solid, and it can interact with an available phonon mode (i.e. something similar to a resonance condition), this photon can be absorbed by the solid and then converted to heat (it is the energy of these vibrations or phonons that we commonly refer to as heat). The solid is then opaque to this particular photon (i.e. at that frequency). Now, unlike the atomic orbitals, the phonon spectrum can be broad and continuous over a large frequency range. That is why all materials have a “bandwidth” of transmission or absorption. The width here depends on how wide the phonon spectrum is.
On the other hand, if a photon has energy beyond the phonon spectrum, then while it can still cause a disturbance of the lattice ions, the solid cannot sustain this vibration, because the phonon mode isn’t available. This is similar to trying to oscillate something at a different frequency than the resonance frequency. So the lattice does not absorb this photon and it is re-emitted but with a very slight delay. This, naively, is the origin of the apparent slowdown of the light speed in the material. The emitted photon may encounter other lattice ions as it makes its way through the material and this accumulates the delay.
Moral of the story: the properties of a solid that we are familiar with have more to do with the “collective” behavior of a large number of atoms interacting with each other. In most cases, these do not reflect the properties of the individual, isolated atoms.
PhD Physics
Accelerator physics, photocathodes, field-enhancement. tunneling spectroscopy, superconductivity






Correct me if I’m wrong but I think that the phonon polariton model described in this article is dominant only at very far infrared frequencies. I believe the dipole interaction with bound electrons becomes dominant at optical frequencies. (I thought a dressed photon state was called a polariton, not an exciton, by the way.)
It might be worth mentioning therefore that this article describes one example of the various ways photons interact with collective excitations?
no
Daz, you are obviously right, I wanted to say "in analogy to phonons" not photons. One can speak of exciton-polaritons and phonon-polaritons.
I don't think that phonons are that important to explain dispersion of light in the visible region as addressed in this article. Much more important are electronic excitations of the atoms and molecules making up the substance. If these excitations are coupled, one speaks of excitons in analogy to photons. In deed the electrons have a much larger oscillator strength as compared with the phonons. Although in transparent media like glass, the eigenfrequencies -or rather broad absorption bands – of these modes lie in the ultraviolet region of the spectrum, they can influence the propagation of light in the visible region. The point is that, though nonresonant, the electric field of the light wave can drive forced polarization of the electron clouds. Now ,- well below the resonance frequency – while the phase of the polarisation will be in phase with the driving field, the electric field radiated by the polarisation will lag behind by 90 degrees. This increasing phase lag is nothing else but a reduction of phase velocity.
Some questions come to my mind.1. Can you try to explain how a mirror actually works?2. Can you expand a bit on translucent materials? Why do some photons get absorbed and others don't? Is the absorption probabilistic, or can it be understood as a cloud of opaque particles in transparent media?