- #1
- 28,947
- 4,240
I am reading an old paper and I have came to the passage shown in the picture:
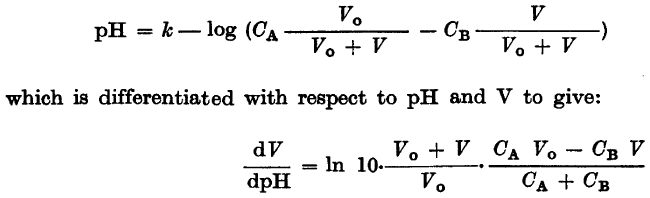
k, CA, CB and V0 are all just some constants.
I think I know what they did - they differentiated the first equation calculating dpH/dV and then took reciprocals of both sides of the equation. Is it a valid approach?
I am not able to get the same result solving the first equation for V and calculating dV/dpH then.
Bear in mind I am mathemathically challenged and English is my second language, I can be missing something obvious.
k, CA, CB and V0 are all just some constants.
I think I know what they did - they differentiated the first equation calculating dpH/dV and then took reciprocals of both sides of the equation. Is it a valid approach?
I am not able to get the same result solving the first equation for V and calculating dV/dpH then.
Bear in mind I am mathemathically challenged and English is my second language, I can be missing something obvious.
Last edited:

