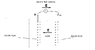- #1
jparke
- 5
- 0
I searched the threads but was unable to find this question:
The electric field inside of a battery points from the positive to the negative terminal, yet when the battery is connected to a conducting loop charge is transported from the negative to the positive terminal as it flows through the circuit. By what mechanism is charge transported *against* the electric field?
(I am treating the current as the flow of positive charge here)
Thanks!
The electric field inside of a battery points from the positive to the negative terminal, yet when the battery is connected to a conducting loop charge is transported from the negative to the positive terminal as it flows through the circuit. By what mechanism is charge transported *against* the electric field?
(I am treating the current as the flow of positive charge here)
Thanks!