- #1
baldbrain
- 236
- 21
Homework Statement
*How many halogens will be removed in the following E2 reactions combined?*
The Attempt at a Solution
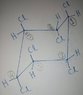
The question's language isn't error free. So, looking at the options, assuming it's *How many halogens will be removed in the following E2 reactions combined?*, the chair comformation of the following compound is:
I know that, for elimination to take place, the chlorines and hydrogens must be anti-periplanar (only possible when both of them are axial). But as you see here, none of the hydrogens turn out to be axial. How does elimination take place then?