- #1
pisluca99
- 63
- 4
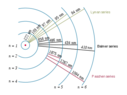
By exciting hydrogen vapors with heat or electrical discharges, it is possible to obtain the element's emission spectrum. In it, as can be seen, appear multiple wavelengths, each corresponding to a particular orbital electronic transition.
From this it can, therefore, be inferred that heat and electrical discharges do not bring a single fixed energy value to the atom, but bring a range of energy values that allow for the different transitions.
How is this possible? Doesn't an electric discharge have a single definite energy value that corresponds to the ∆V between the electrodes? Likewise, does not a given temperature correspond to only one energy value?
From this it can, therefore, be inferred that heat and electrical discharges do not bring a single fixed energy value to the atom, but bring a range of energy values that allow for the different transitions.
How is this possible? Doesn't an electric discharge have a single definite energy value that corresponds to the ∆V between the electrodes? Likewise, does not a given temperature correspond to only one energy value?
Attachments
Last edited: