- #1
Michal Kovac
- 3
- 2
Hi,
up to this day I thought that endothermic and threshold reactions are equivalent. I mean each endothermic reaction must be threshold and each threshold reactions must be endothermic. But I think I was wrong.
Here is example (from this source Q-value):
10B(n,2*alpha)T
This threshold reaction of fast neutron with an isotope 10B is the main way, how radioactive tritium in primary circuit of all PWRs is generated. 10B is the principal source of radioactive tritium in primary circuit of all PWRs (which use boric acid as a chemical shim).
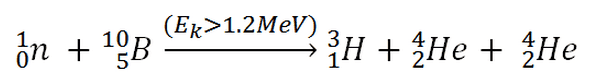
So, this is a threshold reaction and at the same time it is the exothermic reaction, because:
Using the mass-energy equivalence, the Q-value of this reaction is:
Q = {(10.0129+1.00866) [amu] – (3.01604+2 x 4.0026) [amu]} x 931.481 [MeV/amu]
= 0.00036 x 931.481 = 0.335 MeV
Is this consideration right?
I think this reaction is considered to be threshold because of its cross-section:
Figure of reaction cross-section.
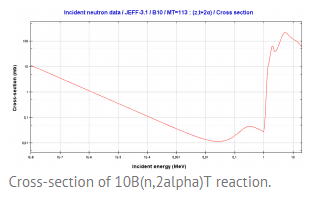
But does anybody know, why the reaction cross-section have a threshold at 1.2 MeV??
Can it be derived from some reaction kinematics or it is "simply" some quantum behaviour of 10B nucleus??
up to this day I thought that endothermic and threshold reactions are equivalent. I mean each endothermic reaction must be threshold and each threshold reactions must be endothermic. But I think I was wrong.
Here is example (from this source Q-value):
10B(n,2*alpha)T
This threshold reaction of fast neutron with an isotope 10B is the main way, how radioactive tritium in primary circuit of all PWRs is generated. 10B is the principal source of radioactive tritium in primary circuit of all PWRs (which use boric acid as a chemical shim).
So, this is a threshold reaction and at the same time it is the exothermic reaction, because:
Using the mass-energy equivalence, the Q-value of this reaction is:
Q = {(10.0129+1.00866) [amu] – (3.01604+2 x 4.0026) [amu]} x 931.481 [MeV/amu]
= 0.00036 x 931.481 = 0.335 MeV
Is this consideration right?
I think this reaction is considered to be threshold because of its cross-section:
Figure of reaction cross-section.
But does anybody know, why the reaction cross-section have a threshold at 1.2 MeV??
Can it be derived from some reaction kinematics or it is "simply" some quantum behaviour of 10B nucleus??