- #1
r12214001
- 24
- 2
- Homework Statement
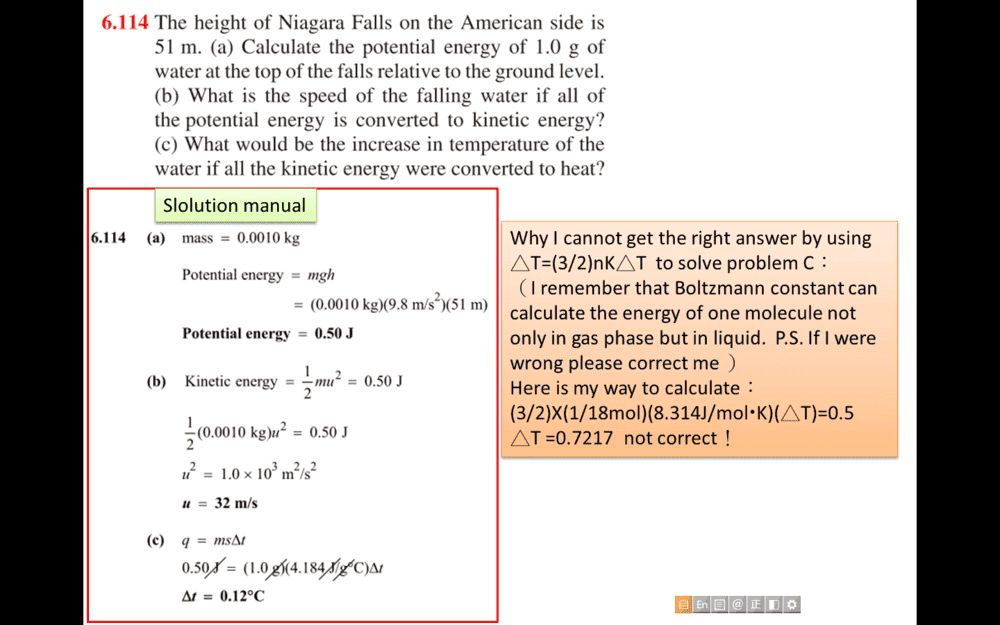
- energy conversion question
- Relevant Equations
- calculate temperature change
Why I cannot get the right answer by using △T=(3/2)nK△T to solve problem C:
Work done at constant pressure is always ##P\Delta V##. And since enthalpy is defined as: H = U + PV, it follows that ##\Delta H = \Delta U + P\Delta V + V\Delta P = \Delta U + P\Delta V## at constant pressure.r12214001 said:As you explaned, W=NKT can only be used in ideal gas.
Why the work for solid Graphite and diamond can be calculated by PV? Because NKT=nRT=PV
concept corrected TKSAndrew Mason said:Work done at constant pressure is always ##P\Delta V##. And since enthalpy is defined as: H = U + PV, it follows that ##\Delta H = \Delta U + P\Delta V + V\Delta P = \Delta U + P\Delta V## at constant pressure.
AM
Energy conversion is the process of transforming energy from one form to another. This can include converting energy from mechanical, thermal, electrical, or chemical forms.
Energy conversion is important because it allows us to harness and utilize different forms of energy for various purposes. For example, converting thermal energy into mechanical energy allows us to power machines and vehicles.
There are several methods of energy conversion, including mechanical, thermal, electrical, and chemical. Mechanical energy conversion involves using machines or devices to convert one form of mechanical energy into another. Thermal energy conversion involves using heat to generate electricity or power machines. Electrical energy conversion involves transforming electrical energy into other forms, such as mechanical, thermal, or chemical. Chemical energy conversion involves using chemical reactions to produce energy.
Energy conversion can have both positive and negative impacts on the environment. The use of renewable energy sources, such as solar or wind power, can help reduce carbon emissions and mitigate the effects of climate change. However, the extraction and conversion of fossil fuels can contribute to air and water pollution and harm ecosystems.
Some examples of energy conversion in everyday life include using a blender to convert electrical energy into mechanical energy to blend food, using a battery to convert chemical energy into electrical energy to power a phone, and using solar panels to convert solar energy into electrical energy to power a home.