- #1
houlahound
- 908
- 223
using alpha decay as a concrete example is the energy of the alpha inside and outside the nucleus the same. it appears so from what I can see but alphas do have a range of energies usually up to between 4 - 9Mev. which is the same range as inside the nucleus.
my question really is does tunnelling for say a particle in a finite box to out of the finite box change the kinetic energy of the particle in any situation, always or never?
the formula for tunnelling probability does not supply info on the energy of the tunnelled particle ie from hyperphysics (simple solution);
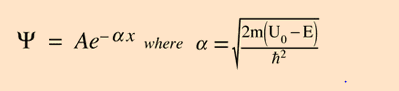
how would one calculate the energy/frequency of the wave function for the tunnelled particle?
my question really is does tunnelling for say a particle in a finite box to out of the finite box change the kinetic energy of the particle in any situation, always or never?
the formula for tunnelling probability does not supply info on the energy of the tunnelled particle ie from hyperphysics (simple solution);
how would one calculate the energy/frequency of the wave function for the tunnelled particle?