- #1
bsodmike
- 82
- 0
First of all, is there a set means for evaluating decay energy be it an alpha, beta, or gamma decay?
Considering a means for evaluating the http://en.wikipedia.org/wiki/Decay_energy" :
[tex]Q=\left(m_0c^2\right)_{\text{after}}-\left(m_0c^2\right)_{\text{before}}[/tex]
I evaluated this disintegration of Tritium into Helium-3:
[tex]^{3}_{1}T\rightarrow~^{3}_{2}He~+~e^-~+~\overline{v}_e[/tex]
Considering the Tritium isotope mass is 3.0160492 u, and Helium-3 isotope mass is 3.0160293 u, [tex]\Delta m=1.99\times 10^{-5}[/tex] u.
Hence, [tex]Q=(1.99\times 10^{-5}\text{u})(939.494~\text{MeV/uc\textsuperscript{2}})(\text{c\textsuperscript{2}})=18.7~\text{keV}[/tex]
I have http://en.wikipedia.org/wiki/Tritium" .
However, considering the following process:
[tex]^{137}_{55}Cs\rightarrow~^{137}_{56}Ba~+~e^-~+~\overline{v}_e[/tex]
This has been explained as,
and as,
Using the earlier method I am obtaining a result of 3.9 MeV if simply considering the rest mass of both caesium-127 (136.91 u) and barium-137 (http://en.wikipedia.org/wiki/Isotopes_of_barium" ). Hence, [tex]Q=(4.188\times 10^{-3}\text{u})(939.494~\text{MeV/uc\textsuperscript{2}})(\text{c\textsuperscript{2}})=3.9~\text{MeV}[/tex]
I can however see that the beta-decay to Ba-137m is simply taken from [tex]E=m_0c^2[/tex] of an electron; thus, [tex]E=(9.11\times 10^{-31}~\text{kg})(3.00\times 10^{8}~\text{ms\textsuperscript{-1}})^2=8.20\times 10^{-14}~\text{J}[/tex]. Since, [tex]1~\text{eV}=1.60\times 10^{-19}~\text{J}[/tex], this results in,
[tex]E=(8.20\times 10^{-14}~\text{J})\left(\dfrac{1~\text{eV}}{1.60\times 10^{-19}~\text{J}}\right)=0.511~\text{MeV}[/tex]
I would appreciate an elucidation of the above problem; thanks in advance to contributors.
Cheers
Mike.
Considering a means for evaluating the http://en.wikipedia.org/wiki/Decay_energy" :
[tex]Q=\left(m_0c^2\right)_{\text{after}}-\left(m_0c^2\right)_{\text{before}}[/tex]
I evaluated this disintegration of Tritium into Helium-3:
[tex]^{3}_{1}T\rightarrow~^{3}_{2}He~+~e^-~+~\overline{v}_e[/tex]
Considering the Tritium isotope mass is 3.0160492 u, and Helium-3 isotope mass is 3.0160293 u, [tex]\Delta m=1.99\times 10^{-5}[/tex] u.
Hence, [tex]Q=(1.99\times 10^{-5}\text{u})(939.494~\text{MeV/uc\textsuperscript{2}})(\text{c\textsuperscript{2}})=18.7~\text{keV}[/tex]
I have http://en.wikipedia.org/wiki/Tritium" .
However, considering the following process:
[tex]^{137}_{55}Cs\rightarrow~^{137}_{56}Ba~+~e^-~+~\overline{v}_e[/tex]
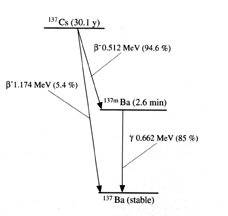
This has been explained as,
Cs137 decays in two ways, (1) beta-decay (maximum energy=0.518 Mev) to Ba137* followed by a gamma-transition to the ground state, and (2) beta-decay (maximum energy=1.2 Mev) directly to the ground state.
and as,
The Cs-137 parent isotope beta decays (~95%) with a 30.17y half-life to produce Ba-137m which in turn decays with a 2.55min. half-live, generating a 661.6 keV gamma ray emission.
Using the earlier method I am obtaining a result of 3.9 MeV if simply considering the rest mass of both caesium-127 (136.91 u) and barium-137 (http://en.wikipedia.org/wiki/Isotopes_of_barium" ). Hence, [tex]Q=(4.188\times 10^{-3}\text{u})(939.494~\text{MeV/uc\textsuperscript{2}})(\text{c\textsuperscript{2}})=3.9~\text{MeV}[/tex]
I can however see that the beta-decay to Ba-137m is simply taken from [tex]E=m_0c^2[/tex] of an electron; thus, [tex]E=(9.11\times 10^{-31}~\text{kg})(3.00\times 10^{8}~\text{ms\textsuperscript{-1}})^2=8.20\times 10^{-14}~\text{J}[/tex]. Since, [tex]1~\text{eV}=1.60\times 10^{-19}~\text{J}[/tex], this results in,
[tex]E=(8.20\times 10^{-14}~\text{J})\left(\dfrac{1~\text{eV}}{1.60\times 10^{-19}~\text{J}}\right)=0.511~\text{MeV}[/tex]
I would appreciate an elucidation of the above problem; thanks in advance to contributors.
Cheers
Mike.
Last edited by a moderator: