- #1
johnk
- 1
- 0
Hey guys! I'm not sure if I solved this correctly. If you could please take a look at my answers I'd really appreciate it. Thank you in advance!
(I tried using the super subscript option but I started to confuse myself because of all the coding so I just stuck with the up arrow (^) I hope you guys don't mind too much) Thanks again
The following figure shows a diagram of an Al/Fe galvanic cell. Use the diagram to
answer the questions that follow it.
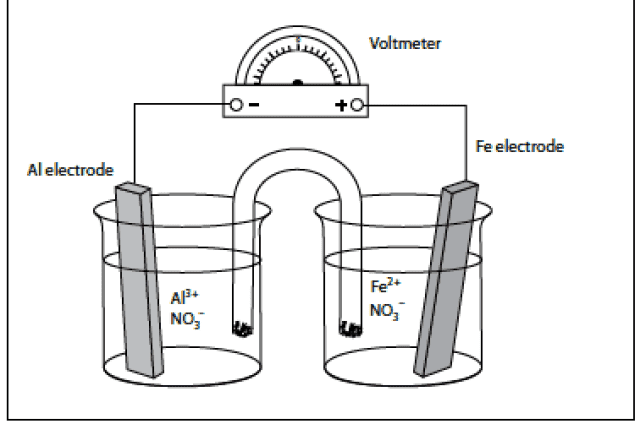
a) Predict the anode, cathode, and overall cell reactions that occur as this cell operates.
b) Predict the overall cell potential.
c) Describe the direction of ion and electron flow in the diagram.
a)
• Fe = Cathode
• Al = Anode
• Al(s) => Al^3+(aq) + 3e^- Fe^2+(aq) + 2e^- => Fe(s)
2(Al => Al^3+ + 3e^-
3(Fe^2+ + 2e^- => Fe)
=2Al + 3Fe^2+ + 6e^- => 2Al^3+ + 6e^- + 3Fe
=2Al + 3Fe^2+ => 2Al^3+ + 3Fe
b)
Al(s) => Al^3+(aq) + 3e^- E=1.66
Fe^2+(aq) + 2e^- => Fe(s) E=-0.41
Overall E= 1.25
c) In an operating galvanic cell, electrons flow through the conducting wire, while ions flow through the solution and salt bridge.
(I tried using the super subscript option but I started to confuse myself because of all the coding so I just stuck with the up arrow (^) I hope you guys don't mind too much) Thanks again
Homework Statement
The following figure shows a diagram of an Al/Fe galvanic cell. Use the diagram to
answer the questions that follow it.
a) Predict the anode, cathode, and overall cell reactions that occur as this cell operates.
b) Predict the overall cell potential.
c) Describe the direction of ion and electron flow in the diagram.
The Attempt at a Solution
a)
• Fe = Cathode
• Al = Anode
• Al(s) => Al^3+(aq) + 3e^- Fe^2+(aq) + 2e^- => Fe(s)
2(Al => Al^3+ + 3e^-
3(Fe^2+ + 2e^- => Fe)
=2Al + 3Fe^2+ + 6e^- => 2Al^3+ + 6e^- + 3Fe
=2Al + 3Fe^2+ => 2Al^3+ + 3Fe
b)
Al(s) => Al^3+(aq) + 3e^- E=1.66
Fe^2+(aq) + 2e^- => Fe(s) E=-0.41
Overall E= 1.25
c) In an operating galvanic cell, electrons flow through the conducting wire, while ions flow through the solution and salt bridge.
 )
)