- #1
Greenybwa
- 5
- 0
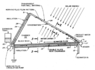
I need to increase the surface area of the glass which will be used in a solar still with the intention of keeping the glass as cool as possible. My first thought was bubble wrap because it's transparent and I thought it would not interfere with the light but then I remembered it is a good insulator and would probably make my glass heat up, so then I was thinking of corrugated glass.
Can someone give me some guidance on other methods to keep the glass cool without using any electricity or any form of human intervention such as pouring water on it.
Thanks
Can someone give me some guidance on other methods to keep the glass cool without using any electricity or any form of human intervention such as pouring water on it.
Thanks