- #1
alex-book
- 9
- 0
Heat transfer problem--please help
I have a heating mantle (180Watt) and i want to use it for heating a ceramics flask (thermal conductivity : 1.38 W/m*K), however, there is a stainless steel on top of the flask (at 10cm distance between the heating mantle and the stainless steel). The thermal conductivity of SS is 21.5 W/m*K. and i know the formula is
h = delta Q/ A* delta T* delta t
If want to know what is the temperature on the stainless steel after it has been heated up for 1 hour, should i calculate the heat transfer between the heating mantle and the flask first? and then go along with the stainless stell or how?
I know Q=(1/((1/h)+(t/k))*A*Delta T
and Q = 180 W
h = specific heat? how could i get this value?
t = 3mm = 0.003m (thickness of the wall)
k = 1.38 W/m*K (thermal conductivity of flask)
A = area of the wall = 17cm*15cm = 255cm^2 = 0.0255m^2
but i don't know what will be the temperature difference between them?
and i also confuse about how could i calculate the heat transfer with open system?
I just need to find what will be the temperature of the stainless steel above 10cm from the heating mantle...
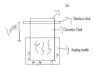
I am wondering that since ceramic is not a good heat conductor, plus the fact that it is an non-isolated system , so the heat would probably not going to be that hot, but still i need to get the approximation...i have put an image of the illustration..
any help will be appreciated..i am really lost at this one..thank you so much!
Alex..
Homework Statement
I have a heating mantle (180Watt) and i want to use it for heating a ceramics flask (thermal conductivity : 1.38 W/m*K), however, there is a stainless steel on top of the flask (at 10cm distance between the heating mantle and the stainless steel). The thermal conductivity of SS is 21.5 W/m*K. and i know the formula is
h = delta Q/ A* delta T* delta t
Homework Equations
If want to know what is the temperature on the stainless steel after it has been heated up for 1 hour, should i calculate the heat transfer between the heating mantle and the flask first? and then go along with the stainless stell or how?
The Attempt at a Solution
I know Q=(1/((1/h)+(t/k))*A*Delta T
and Q = 180 W
h = specific heat? how could i get this value?
t = 3mm = 0.003m (thickness of the wall)
k = 1.38 W/m*K (thermal conductivity of flask)
A = area of the wall = 17cm*15cm = 255cm^2 = 0.0255m^2
but i don't know what will be the temperature difference between them?
and i also confuse about how could i calculate the heat transfer with open system?
I just need to find what will be the temperature of the stainless steel above 10cm from the heating mantle...
I am wondering that since ceramic is not a good heat conductor, plus the fact that it is an non-isolated system , so the heat would probably not going to be that hot, but still i need to get the approximation...i have put an image of the illustration..
any help will be appreciated..i am really lost at this one..thank you so much!
Alex..