- #1
fatcats
- 34
- 1
Homework Statement
See attached file
Homework Equations
N/A
The Attempt at a Solution
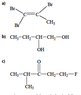
a) 1,1,2-tribromo-1-propene
Now this one I'm not sure about. Is the CH3 a methyl group attached to ethene?
How can I consistently tell the main hydrocarbon chain in a diagram like this?
b) 1,2-butanediol
quite sure this one is right
c) 1-fluoro-4-methyl-3-pentanone
Second least sure about this one