- #1
orgmann
- 4
- 0
Please post this type of questions in HW section using the template.
There is reaction between
PhCHO + SF4
I tried to make the mechanism but I stuck .
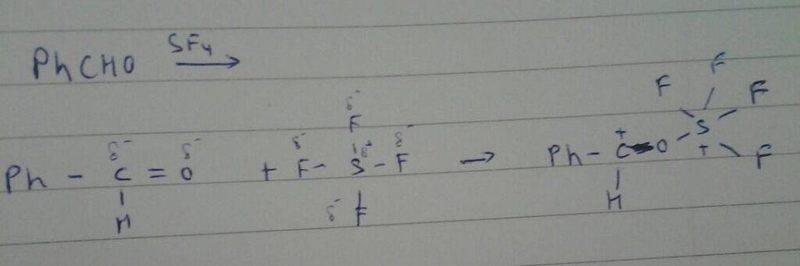
PhCHO + SF4
I tried to make the mechanism but I stuck .
The amount of PhCHO and SF4 needed for the reaction will depend on the specific goals and conditions of the experiment. It is important to carefully consider the stoichiometry of the reaction and the desired concentration of each reagent, as well as any potential side reactions or byproducts that may occur. Consult with a mentor or reference materials to determine the appropriate ratios and amounts for your specific reaction.
The choice of solvent for this reaction will depend on several factors, including the solubility of the reactants and the desired reaction conditions (e.g. temperature, pressure). Common solvents used in this type of reaction include dichloromethane, acetonitrile, and tetrahydrofuran. It is important to choose a solvent that will not interfere with the reaction or produce unwanted side products.
The key to achieving the desired product in this reaction is careful control of the reaction conditions. This includes maintaining the appropriate temperature and concentration of reagents, as well as controlling the reaction time. Additionally, it may be helpful to monitor the reaction progress using techniques such as TLC or NMR. If the desired product is not obtained, it may be necessary to adjust the reaction conditions or try alternative reaction methods.
Both PhCHO and SF4 can be hazardous chemicals, so it is important to take proper safety precautions when handling them. This includes wearing appropriate personal protective equipment, working in a well-ventilated area, and following proper handling and disposal procedures. It is also important to consult safety data sheets and follow any additional safety guidelines provided by your institution or regulatory agencies.
The PhCHO + SF4 reaction can typically be scaled up for larger quantities with careful consideration of the reaction conditions and safety precautions. It is important to consult with a mentor or reference materials to ensure that the appropriate adjustments are made for larger-scale reactions. Additionally, it may be beneficial to perform a small-scale test reaction before scaling up to identify any potential issues or challenges that may arise.