- #1
Sparky42
- 4
- 0
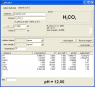
I am trying to perform the calculation that will tell me how much (mass or moles) carbon dioxide (CO2) I would theoretically need to bubble through a 3 liter solution of a 2.6 M aqueous sodium hydroxide (NaOH) solution in order to reduce the pH of the solution to 12. I know that the reaction will be CO2 + NaOH ---> HCO3- + Na+ but I am unaware of how to calculate the mass of carbon dioxide I will need. Any help would be greatly appreciated. Please show all steps of work as I would like to understand this for future knowledge.
Thanks!
Thanks!