- #1
unscientific
- 1,734
- 13
Hi guys, I'm studying the Kinetic Theory of gases from Pauli's book Vol. 3. Here he describes a section on the Mean free path, where the probability of two particles with speed v and v' colliding is described as:
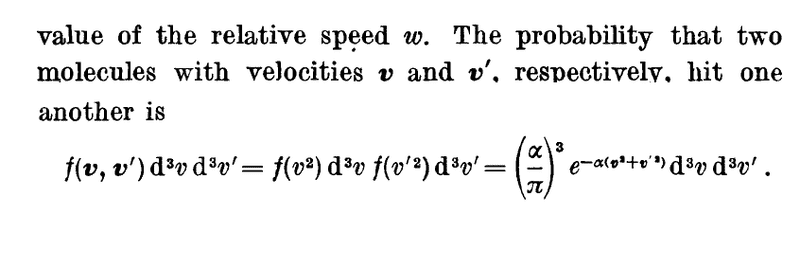 What is f(v,v') here? Is it the velocity distribution function? If so, isn't it simply the fraction of molecules with velocities v and v' ?
What is f(v,v') here? Is it the velocity distribution function? If so, isn't it simply the fraction of molecules with velocities v and v' ?