- #1
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Which Structure Has The Highest Melting Point?
- Thread starter ProblemSets
- Start date
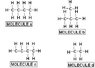
In summary, the conversation discusses the factors that determine a molecule's melting point and which structure would have the highest melting point. The molecular forces, specifically hydrogen, dipole-dipole, and London dispersion, are considered. The individual believes that the answer is C due to its long polar carbon chain, but after further research, concludes that the correct answer is actually A.
Physics news on Phys.org
- #2
Hootenanny
Staff Emeritus
Science Advisor
Gold Member
- 9,623
- 9
Firstly, what molecular features determine a molecule's melting point?
- #3
ProblemSets
- 22
- 0
the forces:
hydrogen
dipoole-dipole
london dispersion
I am thinking C. am i right?
hydrogen
dipoole-dipole
london dispersion
I am thinking C. am i right?
- #4
Hootenanny
Staff Emeritus
Science Advisor
Gold Member
- 9,623
- 9
Your are correct, but do you know why?
- #5
ProblemSets
- 22
- 0
i believe B and C are dipole-dipole forces, which are stronger than A and D which are london disperion forces. i know dipole-diople is stronger than london. Would the answer be C since it has the longest carbon chain that is polar.
thanks
thanks
- #6
chemisttree
Science Advisor
Homework Helper
Gold Member
- 3,943
- 778
Keep working at it... "C" (1-butene) is NOT CORRECT!
- #7
Hootenanny
Staff Emeritus
Science Advisor
Gold Member
- 9,623
- 9
In which case, I stand corrected. I guess I should stick to Math & Physics, and butt out of chem...

- #8
ProblemSets
- 22
- 0
I did some research and i believe that the answer is A.
does that sound right?
does that sound right?
- #9
chemisttree
Science Advisor
Homework Helper
Gold Member
- 3,943
- 778
How did you research your answer?
1. What is the definition of melting point?
The melting point is the temperature at which a solid substance changes into a liquid state.
2. How is the melting point determined?
The melting point is determined by heating a substance until it reaches its melting point, at which point it will begin to melt. The temperature at which this occurs is recorded as the melting point.
3. Why is the melting point of a substance important?
The melting point is important because it is a physical property that can help identify a substance. It can also give an indication of the purity and quality of a substance, as impurities can lower the melting point.
4. What factors affect the melting point of a substance?
The chemical composition, molecular structure, and intermolecular forces of a substance can all affect its melting point. Additionally, external factors such as pressure and impurities can also influence the melting point.
5. Which structure typically has the highest melting point?
Generally, substances with stronger intermolecular forces and more complex molecular structures tend to have higher melting points. Therefore, structures such as diamond and graphite, which have strong covalent bonds, have very high melting points compared to other substances.
Similar threads
-
Classical Physics
- Replies
- 3
- Views
- 1K
-
Biology and Chemistry Homework Help
- Replies
- 5
- Views
- 3K
-
Chemistry
- Replies
- 9
- Views
- 2K
-
Thermodynamics
- Replies
- 4
- Views
- 1K
-
Classical Physics
- Replies
- 11
- Views
- 829
Chemistry
Structural isomer boiling points
-
Biology and Chemistry Homework Help
- Replies
- 5
- Views
- 2K
-
General Engineering
- Replies
- 1
- Views
- 913
-
Electromagnetism
- Replies
- 3
- Views
- 784
-
Biology and Chemistry Homework Help
- Replies
- 1
- Views
- 626
- Replies
- 13
- Views
- 3K
Share: