- #1
duchuy
- 79
- 3
- Homework Statement
- Nucleophilic or electrophilic substitution
- Relevant Equations
- x
Hi,
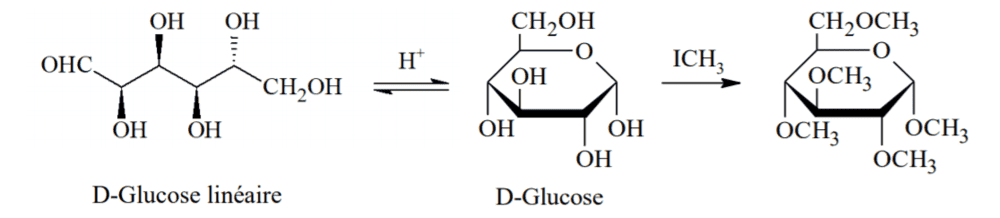
I don't understand why this correction would be a nucleophile substitution?
From what understand : The O atoms in the cyclic glucose acts as nucleophiles. ICH3 is also a nucleophile due to the presence of iodine's "non bonding electron pairs" (sorry I don't know the right term for this), and since I is a halogen, CH3 bares partial positive charge.
Ch3 being electrophilic, comes and replace H. So why isn't it a electrophile substitution?
Can you please explain to me what went wrong in my thought process?
And do you have a way that which I can determine what kind of substitution it is everytime because I don't really know which molecule would be the one that that has a substituted group? Like from this case, is H substituted by CH3 or I substituted by glucose?
Thank you so much for your help!
I don't understand why this correction would be a nucleophile substitution?
From what understand : The O atoms in the cyclic glucose acts as nucleophiles. ICH3 is also a nucleophile due to the presence of iodine's "non bonding electron pairs" (sorry I don't know the right term for this), and since I is a halogen, CH3 bares partial positive charge.
Ch3 being electrophilic, comes and replace H. So why isn't it a electrophile substitution?
Can you please explain to me what went wrong in my thought process?
And do you have a way that which I can determine what kind of substitution it is everytime because I don't really know which molecule would be the one that that has a substituted group? Like from this case, is H substituted by CH3 or I substituted by glucose?
Thank you so much for your help!