- #1
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Are Incorrect Bond Angles Common in Organic Chemistry?
- Thread starter alingy1
- Start date
-
- Tags
- Mistake
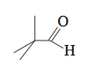
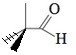
In summary, the conversation discusses the issue of incorrect bond angles in a picture of C5H10O and whether or not it is common in organic chemistry to disregard bond angles when drawing molecules as projections. The expert clarifies that projections are often used in organic chemistry and that they may not always perfectly represent the true spatial arrangement of atoms.
Physics news on Phys.org
- #2
ChiralWaltz
- 161
- 5
The two methyl groups pointing down on the left should consist of a dash and a wedge, giving it the bond angle of 109.5 degrees. It could be a really awful Fisher projection also :)
- #3
Borek
Mentor
- 28,947
- 4,240
alingy1 said:Please look at picture. It seems as though the bond angles are wrong for C5H10O. Is it common in organic chemistry to not respect bond angles?
I had to think for a moment to spot the problem - it was so obvious to me what the left part if the image is intended to mean (see ChiralWaltz post), that it required an effort to see it as 2D.
It is common in organic chemistry to draw molecules as projections, as they are (almost) never flat. Dashes and edges are used only when the molecule drawn without them is ambiguous. Here, when you know it is a projection, it doesn't matter which carbon goes up and which goes down. On top on that, these projections are almost never perfect (there is no right angle in the molecule), but are accepted as long as it is clear what is the intended spatial arrangement of the atoms.
- #4
- #5
DeeNos
- 1,118
- 0
Thank you for bringing this to my attention. It is not uncommon for mistakes to occur in bondline structures, especially in complex molecules such as C5H10O. In organic chemistry, it is important to respect bond angles as they determine the 3-dimensional shape and properties of a molecule. However, human error and technical limitations can sometimes lead to incorrect bond angles being depicted. It is important to always double check and verify bond angles in chemical structures to ensure accuracy in representation. Thank you again for pointing out this mistake.
1. What is a mistake in aldehyde bondline?
A mistake in aldehyde bondline refers to an error in the way the bonds are depicted in the structural formula of an aldehyde molecule. This can include incorrect placement of bonds, missing or extra bonds, or incorrect representation of the molecule's structure.
2. How can a mistake in aldehyde bondline affect the accuracy of a chemical reaction?
A mistake in aldehyde bondline can greatly impact the accuracy of a chemical reaction. This is because the bonds depicted in the structural formula determine the spatial arrangement of the atoms in the molecule, which in turn affects the molecule's reactivity and behavior in a reaction.
3. What are some common causes of mistakes in aldehyde bondline?
Mistakes in aldehyde bondline can be caused by human error, lack of understanding of chemical structures, and misinterpretation of structural formulas. They can also be a result of software or computer errors when creating digital representations of molecules.
4. How can mistakes in aldehyde bondline be avoided?
To avoid mistakes in aldehyde bondline, it is important to have a thorough understanding of chemical structures and how to properly depict them. Double-checking the bonds and structure before drawing a structural formula can also help catch any errors. Additionally, using reliable and accurate software or tools for creating structural formulas can help prevent mistakes.
5. What are the consequences of not correcting a mistake in aldehyde bondline?
If a mistake in aldehyde bondline is not corrected, it can lead to incorrect interpretations of the molecule's properties and reactivity. This can have serious consequences in a laboratory setting, where accurate representations of molecules are crucial for conducting experiments and predicting outcomes. It can also lead to errors in research and data analysis, which can hinder scientific progress.
Similar threads
-
Biology and Chemistry Homework Help
- Replies
- 5
- Views
- 3K
-
Biology and Chemistry Homework Help
- Replies
- 1
- Views
- 613
-
Biology and Chemistry Homework Help
- Replies
- 1
- Views
- 3K
-
Biology and Chemistry Homework Help
- Replies
- 6
- Views
- 2K
-
Biology and Chemistry Homework Help
- Replies
- 4
- Views
- 9K
-
STEM Academic Advising
- Replies
- 2
- Views
- 1K
-
Biology and Chemistry Homework Help
- Replies
- 1
- Views
- 4K
-
Biology and Chemistry Homework Help
- Replies
- 4
- Views
- 3K
-
Biology and Chemistry Homework Help
- Replies
- 3
- Views
- 1K
- Replies
- 10
- Views
- 1K
Share: