- #1
etotheipi
Two different labelling schemes for MOs are shown on the left and right of this diagram:
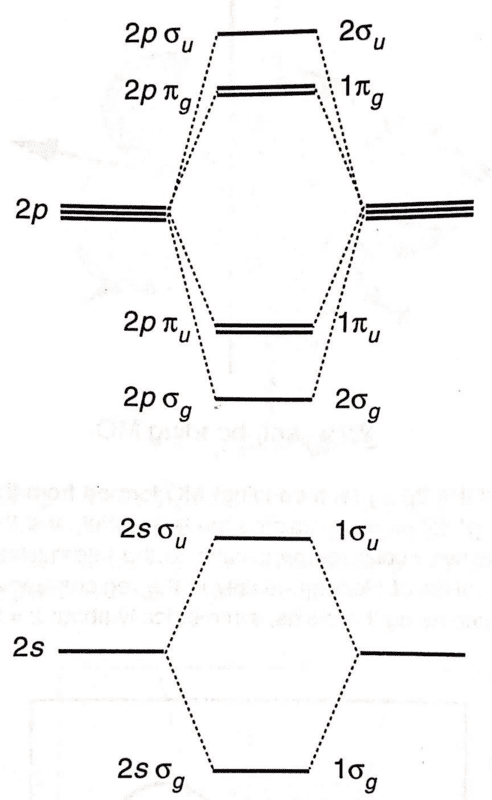
I understand the LHS naming scheme, since the MOs are simply labelled according to the AOs from which they are derived (along with ##\sigma##/##\pi## and ##u##/##g## for the MO).
I don't understand the numbering scheme on the RHS. The book says that 'orbitals of the same symmetry are distinguished from one another by numbering them 1,2,...'. What does this mean? Thanks.
I understand the LHS naming scheme, since the MOs are simply labelled according to the AOs from which they are derived (along with ##\sigma##/##\pi## and ##u##/##g## for the MO).
I don't understand the numbering scheme on the RHS. The book says that 'orbitals of the same symmetry are distinguished from one another by numbering them 1,2,...'. What does this mean? Thanks.