- #1
Lebnm
- 31
- 1
We can use the method of the Green functions to calculate ioniation potentials and electron affinities of atoms and molecules. These quantities can be determined if we know the self-energy ##\Sigma(E)##, that is a function of the energy ##E##. A matrix element of the self-energy in the basis of spin-orbitals has a spectral form $$\Sigma_{pq}(E) = \Sigma_{pq}(\infty) + \sum_{\mu}\frac{f_{pq\mu}}{E - M_{\mu} + i\eta} + \sum_{\nu}\frac{g_{pq\nu}}{E - N_{\nu} - i\eta},$$where ##\Sigma_{pq}(\infty)## is a E-independent term and ##\eta## is an infinitesimal positive. We can see that the self-energy has poles in the sets ##\{M_{\mu}\}## and ##\{N_{\nu}\}##, but this poles are separeted by a interval ##E = E_{0}##. It means that there aren't poles of the first sum between two poles of the second sum, and vice-versa. If we call the poles of the first sum by ##\Sigma_{+1},\ \Sigma_{+2},\ ...## in the increasing order and the poles of the second sum in decreasing order by ##\Sigma_{-1},\ \Sigma_{-2},\ ...##, and plot a graph of the self-energy, we espect that it has a form like:
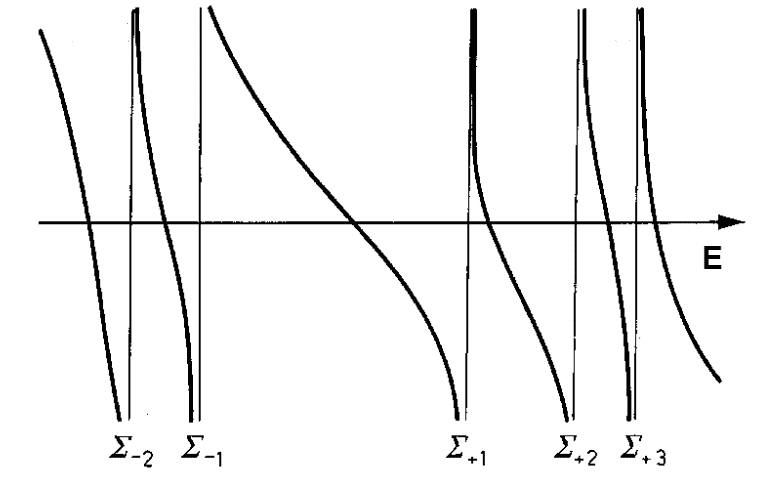
The article I am reading say that, to a closed-shell system, the interval between the poles ##\Sigma_{-1}## and ##\Sigma_{+1}## conteim the energies of the outer valence electrons. He say that is easy to see looking to the perturbation expansion of the self-energy, that is similar to the expansion of the Green function using Feynman diagrams, but I can't see why. Does anyone have any idea?
The article I am reading say that, to a closed-shell system, the interval between the poles ##\Sigma_{-1}## and ##\Sigma_{+1}## conteim the energies of the outer valence electrons. He say that is easy to see looking to the perturbation expansion of the self-energy, that is similar to the expansion of the Green function using Feynman diagrams, but I can't see why. Does anyone have any idea?
