- #1
Eva Brain
- 22
- 0
I am learning Chemistry from one book and I have bumped onto a following image with given tasks:
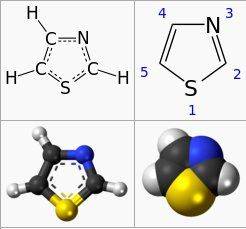
What is the balanced reaction?
What is the RMM of thiazole?
What is the fuel-air mass ratio?
What is the oxygen depletion?
What is the yield of CO2?
What is the yield of H2O?
What is the yield of N2?
For ideal combustion, what is the yield of carbon-monoxide?
Now, aformentioned product is Thiazole( checked on wiki ) and products of combustion are carbon dioxide, water, hydrogen sulphide (H2S) and nitrogen. Now how can I solve this matter having in mind following data that air is consisted of 21% O2 and 79% of N2.
What is the balanced reaction?
What is the RMM of thiazole?
What is the fuel-air mass ratio?
What is the oxygen depletion?
What is the yield of CO2?
What is the yield of H2O?
What is the yield of N2?
For ideal combustion, what is the yield of carbon-monoxide?
Now, aformentioned product is Thiazole( checked on wiki ) and products of combustion are carbon dioxide, water, hydrogen sulphide (H2S) and nitrogen. Now how can I solve this matter having in mind following data that air is consisted of 21% O2 and 79% of N2.