- #1
nhk150709
- 3
- 0
I have no idea how to solve these problems.
Picture is attached.
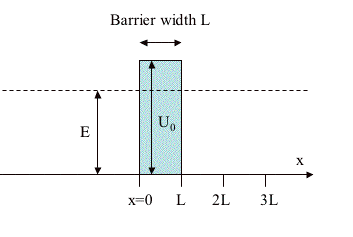
An electron with energy E is incident on a barrier of height U0 and total width L. It enters the barrier at x = 0, from the left.
1) For an electron with energy E = 6 eV and a barrier of height U0 = 12 eV, at what penetration depth into the barrier will the probability density fall to 1/4 of its value at the surface x = 0 ? Assume the barrier width L is sufficiently large that the in-barrier wavefunction is well approximated by a single exponential, i.e., negligible reflections from the x = L interface. [For an electron, mec2 = 0.511 MeV.]
0.055 nm
0.110 nm
0.282 nm
0.159 nm
0.319 nm
2) What is the probability density for this electron at the depth 3 times as large as the answer to the previous question, compared to its value at x = 0 ? Assume that the electron is still inside the barrier at this depth.
1/12
(1/4) 2
(1/4) 35) For the same barrier width L, if the height of the barrier and the energy of the electron are both reduced by a factor of 4, U1 = 0.25 U0 and E1 = 0.25 E, what is the transmission coefficient T1?
T1 = 10-8/4= 0.25 x 10-8
T1 = 10-8
T1 = 4 x 10-8
T1 = 2 x 10-4
The problem does not provide enough information to answer this question.
Picture is attached.
An electron with energy E is incident on a barrier of height U0 and total width L. It enters the barrier at x = 0, from the left.
1) For an electron with energy E = 6 eV and a barrier of height U0 = 12 eV, at what penetration depth into the barrier will the probability density fall to 1/4 of its value at the surface x = 0 ? Assume the barrier width L is sufficiently large that the in-barrier wavefunction is well approximated by a single exponential, i.e., negligible reflections from the x = L interface. [For an electron, mec2 = 0.511 MeV.]
0.055 nm
0.110 nm
0.282 nm
0.159 nm
0.319 nm
2) What is the probability density for this electron at the depth 3 times as large as the answer to the previous question, compared to its value at x = 0 ? Assume that the electron is still inside the barrier at this depth.
1/12
(1/4) 2
(1/4) 35) For the same barrier width L, if the height of the barrier and the energy of the electron are both reduced by a factor of 4, U1 = 0.25 U0 and E1 = 0.25 E, what is the transmission coefficient T1?
T1 = 10-8/4= 0.25 x 10-8
T1 = 10-8
T1 = 4 x 10-8
T1 = 2 x 10-4
The problem does not provide enough information to answer this question.
Attachments
Last edited:
