- #1
sameeralord
- 662
- 3
Hello everyone,
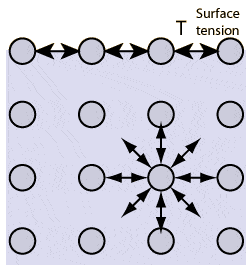
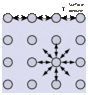
Ok I know surfactant has a polar and non polar group and then go to the surface and break the bonds. My question is the surfactant would still arrange itself like this picture, meaning surfactant and surfactant would have strong bonds at the surface. So how does this reduce surface tension, is surfactant surfactant bond less strong than eg water water bond here? Thanks
Ok I know surfactant has a polar and non polar group and then go to the surface and break the bonds. My question is the surfactant would still arrange itself like this picture, meaning surfactant and surfactant would have strong bonds at the surface. So how does this reduce surface tension, is surfactant surfactant bond less strong than eg water water bond here? Thanks