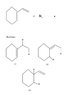
- #1
utkarshakash
Gold Member
- 854
- 13
Homework Statement
Give all possible products of the following reaction. How many stereoisomers of each product could be obained
Homework Equations
See attached image
The Attempt at a Solution
For product 1, there is 1 chiral centre and 1 double bond. ∴No. of products = 22
For 2, it will be same as 1.
For 3, two chiral centres. Thus, 22 products.
Correct answer according to solution:-
1 - 2 products
2 - 4 products
3 - 2 products