- #1
olee
- 24
- 0
found this on this page
http://www.chemguide.co.uk/mechanisms/nucadd/reduce.html#top
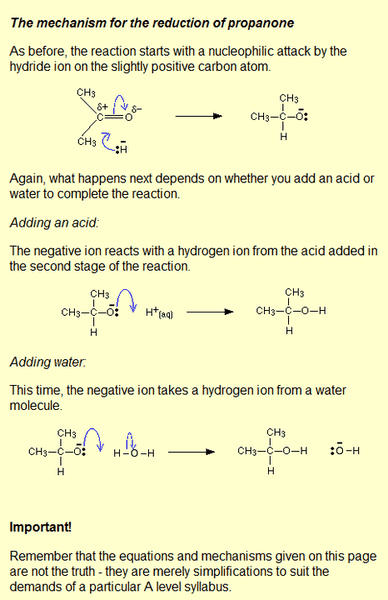
can someone explain why it is simplified? and how does it really happen?
http://www.chemguide.co.uk/mechanisms/nucadd/reduce.html#top
can someone explain why it is simplified? and how does it really happen?