- #1
Manasan3010
- 38
- 3
I suppose the reason I am able to see myself in mirror and not in wood is the reflection off a mirror is specular whereas wood is diffuse.
In reflective material(e.g: silver) when the photon hits the frontline atom, the atom's electrons absorbs the energy and release it back with tiny energy loss.
My question is why the atom re-releasing the absorbed energy in same angle at it got hit? Why it didn't release the photon in a random direction or to the back side?
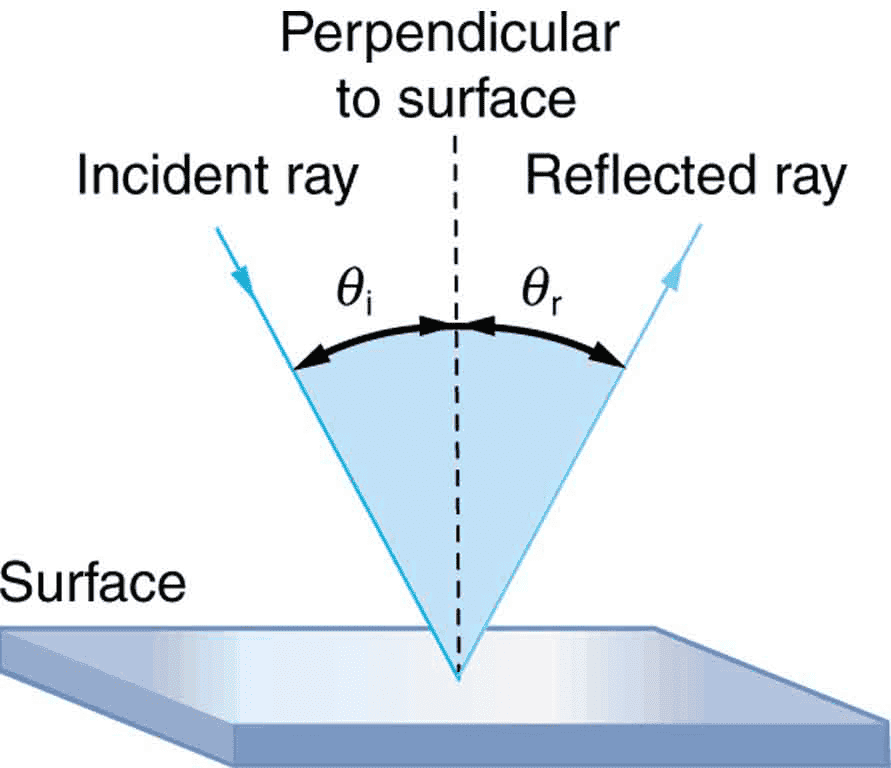
In reflective material(e.g: silver) when the photon hits the frontline atom, the atom's electrons absorbs the energy and release it back with tiny energy loss.
My question is why the atom re-releasing the absorbed energy in same angle at it got hit? Why it didn't release the photon in a random direction or to the back side?