- #1
Raza
- 203
- 0
Using Hess's Law in 2 Questions
Hi, can someone just check my work and see if I did something wrong?
The answers seems strange.
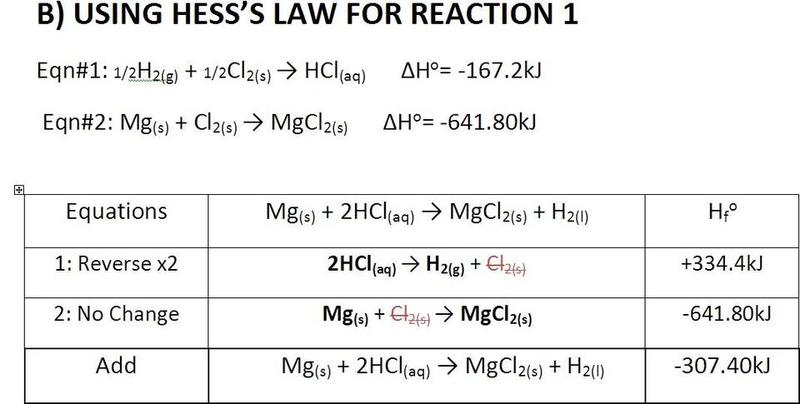
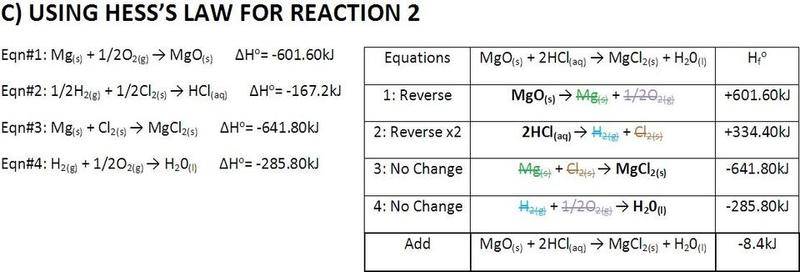
And someone tell me where I can get the enthalpy values of molecules?
Thanks.
Hi, can someone just check my work and see if I did something wrong?
The answers seems strange.
And someone tell me where I can get the enthalpy values of molecules?
Thanks.
Last edited: