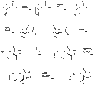- #1
SplinterIon
- 17
- 0
Hey y'all
I'm wondering whether it's possible for the following synthesis to occur and how?:
http://harishkukreja.neargeek.net/Synthesis.jpg
The thing is, after bashing my head against it for about 2 hrs - I've really gotten nowhere . A few of the rxn's we're allowed to use are:
. A few of the rxn's we're allowed to use are:
Hydrogenation (except you can't undo it :grumpy: )
Halogenation + De
Hydrohalogenation + De
Oxidation (KMnO4)
Ozonation (O3)
Esterification
Amide formation
Reduction (LiAlH4 and/or NaBH4).
Hydration + De (H2SO4 conc/dilu.)
Using dehydration + 2*oxidation + esterification I can change the double bond to the bottom chain. using a similar process also yields the top (though in an isomer form). I'm however badly stuck when dealing with the middle section - further the double reactive sites are also bugging me - and blocking isn't really helping me.
Any help would be appreciated and I will worship you forever
I'm wondering whether it's possible for the following synthesis to occur and how?:
http://harishkukreja.neargeek.net/Synthesis.jpg
The thing is, after bashing my head against it for about 2 hrs - I've really gotten nowhere
 . A few of the rxn's we're allowed to use are:
. A few of the rxn's we're allowed to use are:Hydrogenation (except you can't undo it :grumpy: )
Halogenation + De
Hydrohalogenation + De
Oxidation (KMnO4)
Ozonation (O3)
Esterification
Amide formation
Reduction (LiAlH4 and/or NaBH4).
Hydration + De (H2SO4 conc/dilu.)
Using dehydration + 2*oxidation + esterification I can change the double bond to the bottom chain. using a similar process also yields the top (though in an isomer form). I'm however badly stuck when dealing with the middle section - further the double reactive sites are also bugging me - and blocking isn't really helping me.
Any help would be appreciated and I will worship you forever