- #1
mythcism
- 4
- 0
Can somebody explain in detail what causes the peakes.
this is carbon
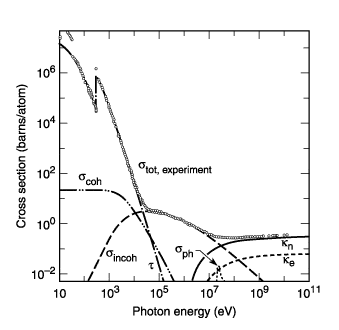
this is lead
( Total photon cross section in carbon, as a function of energy, showing the contributions of different processes: t, atomic photo-effect (electron ejection, photon absorption); , coherent scattering (Rayleigh scattering—atom neither ionized nor excited); , incoherent scattering (Comp- ton scattering off an electron); , pair production, nuclear field; , pair production, electron field; , photonuclear absorption (nuclear absorption, usually followed by emission of a neutron or other particle).
thanks
(btw I am not really familiar with al these terms)
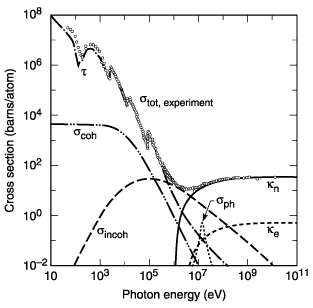
this is carbon
this is lead
( Total photon cross section in carbon, as a function of energy, showing the contributions of different processes: t, atomic photo-effect (electron ejection, photon absorption); , coherent scattering (Rayleigh scattering—atom neither ionized nor excited); , incoherent scattering (Comp- ton scattering off an electron); , pair production, nuclear field; , pair production, electron field; , photonuclear absorption (nuclear absorption, usually followed by emission of a neutron or other particle).
thanks
(btw I am not really familiar with al these terms)