Discussion Overview
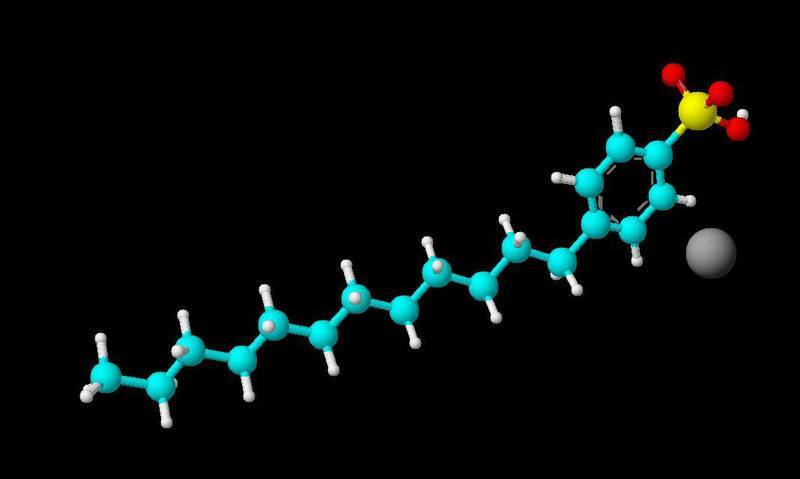
The discussion revolves around the nature of the bond in sodium dodecylbenzene sulfonate, specifically focusing on whether the sodium ion is involved in an ionic bond and how it relates to the overall structure of the compound. The scope includes conceptual clarification and technical explanation of bonding types.
Discussion Character
- Conceptual clarification
- Technical explanation
Main Points Raised
- One participant expresses confusion about the bonding in sodium dodecylbenzene sulfonate and questions whether the sodium ion forms an ionic bond.
- Another participant clarifies that there are both ionic and covalent bonds in the compound, suggesting that the sodium ion does not have a physical bond to the rest of the structure.
- A later reply emphasizes that an ionic bond is a legitimate physical bond, noting that the sodium ion is bonded to one of the oxygen atoms in the sulfonate group, despite the absence of a "stick" representation in the structure.
- One participant acknowledges the explanation and confirms the understanding that the sodium ion does not require a physical "stick" to be considered bonded.
Areas of Agreement / Disagreement
Participants generally agree that the sodium ion is involved in an ionic bond, but there is some confusion regarding the representation of this bond and the nature of physical bonding compared to covalent bonds. The discussion reflects differing interpretations of how ionic bonds are visually represented in molecular structures.
Contextual Notes
There are limitations in the discussion regarding the assumptions about bond representation and the definitions of physical bonds. The conversation does not resolve the nuances of how ionic and covalent bonds are depicted in molecular diagrams.