- #1
Kior
- 11
- 0
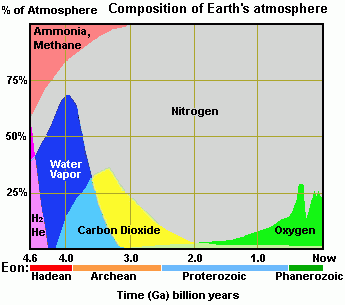
I noticed that the Co2 is actually decreasing in the Eon time.
My guess is lush vegetations or forests began to grow which absorbed the Co2 by photosynthesis? Would anyone give me any clue?
That is a self referential question. If it was not, you would not be here to ask the question.rootone said:Is there something unique about this planet which makes it, well a rocky planet with a mostly N2 atmosphere?.
rootone said:Still, pretty much N2 all the way.
Is there something unique about this planet which makes it, well a rocky planet with a mostly N2 atmosphere?.
From a powerpoint I downloaded from Umich AOSS department site. It seems to be made by a UM professorBaluncore said:The creation of carbonate, oil, and coal deposits can account for a significant reduction in CO2.
We have only had algae and plants in the last 1Ga so that does not explain the big initial drop.
I think it would be quite difficult to get the data needed to draw the graph you show.
The graph also looks like it is based on a hypothesis, not real data.
Where is the graph from?
http://www.space.com/18527-venus-atmosphere.htmlThe atmosphere of Venus is made up almost completely of carbon dioxide. Nitrogen exists in small doses, as do cloudsof sulfuric acid. The air of Venus is so dense that the small traces of nitrogen are four times the amount found on Earth, although nitrogen makes up more than three-fourths of the terrestrial atmosphere
Ironically, the most Earth-like atmosphere in the solar system occurs 30 to 40 miles (50 to 60 kilometers) above the surface of Venus. Both oxygen and hydrogen rise above the heavier gas layer covering the ground, and the pressures are similar to our planet.
- Carbon dioxide: 96 percent
- Nitrogen: 3.5 percent
- Carbon monoxide, argon, sulfur dioxide, and water vapor: less than 1 percent
256bits said:A more correct graph would be like this:
256bits said:
D H said:That said, it's widely accepted that CO2 levels fell from an initially high value prior to the evolution of photosynthesis. CO2 dissolves in water. Some of that dissolved carbon dioxide became carbonic acid, which combined with rock to form carbonate rock.
Who wrote anything about extrapolation?stevmg said:"Extrapolation?" Duhhhh!
So you are claiming that all the data collected indicating rising levels of CO2 over time, is false either due to bad measurements, or it has been falsified deliberately?David Scalone said:Some silicates also absorb CO2, Volcanic sources provide tons of it, as well as CO2. New research indicates more absorption than production, over time.
phyzguy said:What is the Y-axis? Is it %? If so, why does it show the atmosphere to be over 50% O2? This is not correct - it is about 21% O2.
Baluncore said:The creation of carbonate, oil, and coal deposits can account for a significant reduction in CO2.
We have only had algae and plants in the last 1Ga so that does not explain the big initial drop.
rootone said:Still, pretty much N2 all the way.
Is there something unique about this planet which makes it, well a rocky planet with a mostly N2 atmosphere?.
rootone said:A predominantly N2 atmosphere is, I think, unique in this solar system,
CO2 levels have varied throughout Earth's history due to a variety of natural factors, including changes in volcanic activity, ocean circulation patterns, and the Earth's position in its orbit around the sun. Additionally, the evolution of plant life has played a role in regulating CO2 levels through photosynthesis and carbon burial.
Scientists can study past CO2 levels by analyzing air bubbles trapped in ice cores, sediment layers, and fossilized plant material. These methods provide a record of CO2 levels dating back hundreds of thousands of years.
Decreasing CO2 levels can have a cooling effect on the Earth's climate, as CO2 is a greenhouse gas that helps trap heat in the atmosphere. This can lead to changes in global temperature, weather patterns, and sea level.
No, there have been periods in Earth's history where CO2 levels have increased due to natural factors such as increased volcanic activity or changes in ocean circulation. However, the current rate of CO2 increase is much higher than natural fluctuations and is primarily caused by human activities such as burning fossil fuels.
While it is possible for CO2 levels to decrease naturally, it is difficult to reverse the current decrease in CO2 levels caused by human activities. However, reducing our carbon footprint through sustainable practices and transitioning to renewable energy sources can help slow down the decrease and mitigate its impact on the Earth's climate.