- #1
Kalus
- 37
- 0
I have a system that looks like this:
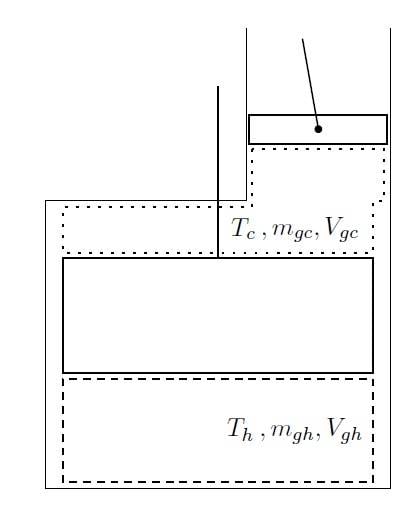
The top part is a piston, whereas the bottom is a displacer.
I have looked at the Isothermal case for this system in a separate thread (https://www.physicsforums.com/showthread.php?t=553165)
But in short, the result was that the pressure of the system is equal to:
[tex]m=m_{gc}+m_{gh}[/tex]
[tex]P=\frac{mR}{V_{gc}/T_c +V_{gh}/T_h}[/tex]
How can I modify this to take into account the temperature rise caused by adiabatic compression? I suppose I need to write the T_gc + T_gh as functions of the compression by the top piston, but how?
The top part is a piston, whereas the bottom is a displacer.
I have looked at the Isothermal case for this system in a separate thread (https://www.physicsforums.com/showthread.php?t=553165)
But in short, the result was that the pressure of the system is equal to:
[tex]m=m_{gc}+m_{gh}[/tex]
[tex]P=\frac{mR}{V_{gc}/T_c +V_{gh}/T_h}[/tex]
How can I modify this to take into account the temperature rise caused by adiabatic compression? I suppose I need to write the T_gc + T_gh as functions of the compression by the top piston, but how?