krackers
- 72
- 0
I was reading this website (http://www.chembuddy.com/?left=pH-calculation&right=pH-amphiprotic-salt) on calculating pH of amphiprotic salts.
My question is why can't you do something similar for diprotic acids? Take for example the diprotic acid H2S.
Because you have the following reactions:
H+ + HS- ⇔ H2S
HS- ⇔ H+ + S2-
can't you treat HS- like an amphiprotic substance and calculate the pH the same way you calculated pH for other amphiprotic substances?
—————————————————————
I also have another question relating to these topics. In this image,
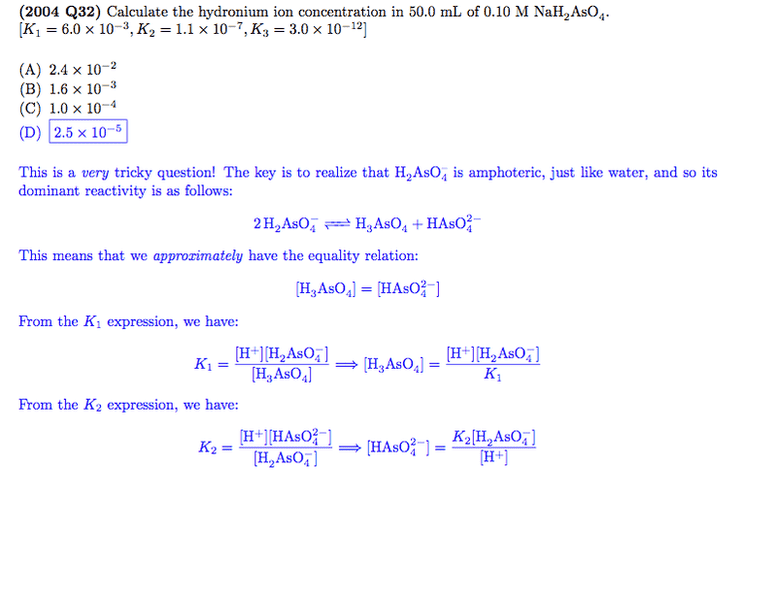
I don't understand how they came to the conclusion that [H3AsO4-] ≈ [HAsO42-]. Further, why is H2AsO42- considered amphiprotic when the value of K-1, that is the equilibrium constant for H2AsO42- + H+ ⇔ H3AsO4, is much higher than that of the reaction where it acts an acid?
My question is why can't you do something similar for diprotic acids? Take for example the diprotic acid H2S.
Because you have the following reactions:
H+ + HS- ⇔ H2S
HS- ⇔ H+ + S2-
can't you treat HS- like an amphiprotic substance and calculate the pH the same way you calculated pH for other amphiprotic substances?
—————————————————————
I also have another question relating to these topics. In this image,
I don't understand how they came to the conclusion that [H3AsO4-] ≈ [HAsO42-]. Further, why is H2AsO42- considered amphiprotic when the value of K-1, that is the equilibrium constant for H2AsO42- + H+ ⇔ H3AsO4, is much higher than that of the reaction where it acts an acid?