Qube
Gold Member
- 461
- 1
My teacher insists the name of this compound is (2S)-chloro-3-methyl-3-propylhexane
Shouldn't it be 4-(1-chloroethyl)-4-methylheptane? (Ignore R,S configuration for now).
Chemoffice also tells me 4-(1-chloroethyl)-4-methylheptane.
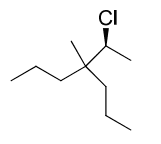
I think it's the latter because I've consulted three textbooks; all say pick out the longest chain of carbon atoms.
I've also consulted Wikipedia; it says pick the parent chain with the following rules in mind (in descending order of importance):
1) Pick the most substituted chain - the chain with the most substituents of the parent suffix ("ane") - so that would mean either the hexane or the heptane chain, right? The hexane chain has a methyl and an propyl substituent, and a chloro substituent (it's a halogen, however, so it doesn't count). The heptane chain has a complex alkyl substituent and a methyl substituent too.
2) Pick the longest chain. So the heptane chain should win here.
Shouldn't it be 4-(1-chloroethyl)-4-methylheptane? (Ignore R,S configuration for now).
Chemoffice also tells me 4-(1-chloroethyl)-4-methylheptane.
I think it's the latter because I've consulted three textbooks; all say pick out the longest chain of carbon atoms.
I've also consulted Wikipedia; it says pick the parent chain with the following rules in mind (in descending order of importance):
1) Pick the most substituted chain - the chain with the most substituents of the parent suffix ("ane") - so that would mean either the hexane or the heptane chain, right? The hexane chain has a methyl and an propyl substituent, and a chloro substituent (it's a halogen, however, so it doesn't count). The heptane chain has a complex alkyl substituent and a methyl substituent too.
2) Pick the longest chain. So the heptane chain should win here.
Last edited: