Rainbows_
Hi,
The following is the typical raman spectra of water.
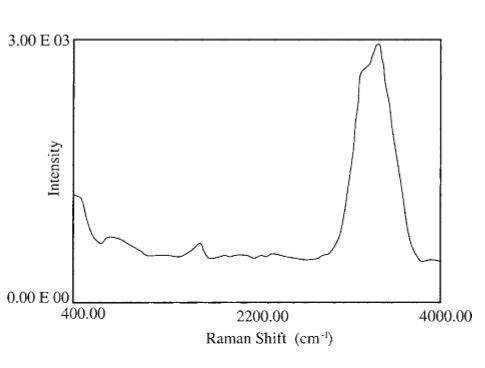
In their normal form, water molecules maintain a simple configuration: an oxygen atom is in the center and two hydrogen atoms are on the two sides symmetrically. Under ambient conditions, water molecules within liquid water give rise to a strong Raman shift peak at 3,430 cm^-1 and a weaker peak at 1,635 cm^-1, respectively.
The background laser Raman spectra showed that there is a stretching vibrational peak for OH at 3,410 cm^-1, and a deformed and weak vibrational peak for HOH at 1,635 cm^-1 at about 12 C.
Now look at the following Raman spectra of the same water.
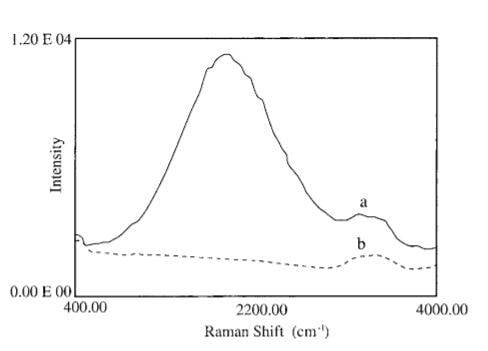
Notice the 15 times bigger raman shift centered at 2100 cm^-1 and the spectral intensity of the peak is about 15 times higher than the strong hydrogen stretching peak at 3,430 cm..
Does anyone know what can cause such huge raman shifts.. what do you need to do to water before it can cause such effect for instance? And what part of the molecules are affected? What is your opinion?
The following is the typical raman spectra of water.
In their normal form, water molecules maintain a simple configuration: an oxygen atom is in the center and two hydrogen atoms are on the two sides symmetrically. Under ambient conditions, water molecules within liquid water give rise to a strong Raman shift peak at 3,430 cm^-1 and a weaker peak at 1,635 cm^-1, respectively.
The background laser Raman spectra showed that there is a stretching vibrational peak for OH at 3,410 cm^-1, and a deformed and weak vibrational peak for HOH at 1,635 cm^-1 at about 12 C.
Now look at the following Raman spectra of the same water.
Notice the 15 times bigger raman shift centered at 2100 cm^-1 and the spectral intensity of the peak is about 15 times higher than the strong hydrogen stretching peak at 3,430 cm..
Does anyone know what can cause such huge raman shifts.. what do you need to do to water before it can cause such effect for instance? And what part of the molecules are affected? What is your opinion?