Discussion Overview
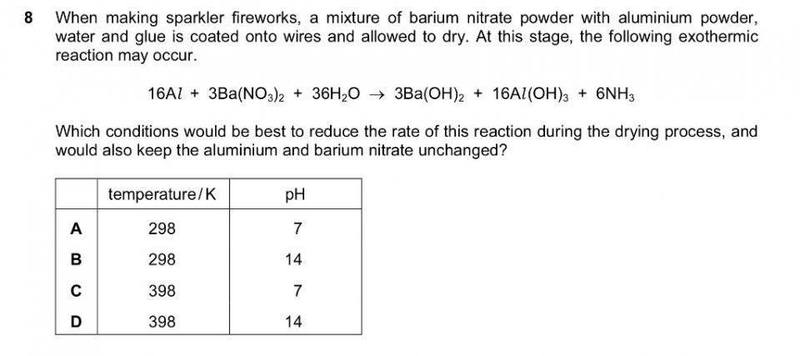
The discussion revolves around the chemical reaction between aluminum and barium nitrate, focusing on the stability of aluminum powder in various pH environments and the implications of pH on the reaction dynamics. Participants explore concepts related to reactivity, protonation, and the behavior of aluminum hydroxide in different solutions.
Discussion Character
- Homework-related
- Technical explanation
- Conceptual clarification
- Debate/contested
Main Points Raised
- One participant suggests that adding more OH- would shift the reaction to the left and questions why pH 7 is the answer.
- Another participant notes that aluminum powder is relatively stable due to the formation of an oxide layer, which prevents vigorous reactions with air oxygen.
- Some participants discuss how aluminum's lowered reactivity is affected by neutral, acidic, and caustic solutions.
- There is a suggestion that the explanation should involve the protonation of aluminum hydroxide and the volatility effects on ammonia.
- A participant expresses uncertainty about the clarity of the explanation and emphasizes the need for explicitness in discussing the reactions involved.
- Another participant mentions the deprotonation of aluminum hydroxide as pH changes from 7 to 14 and the behavior of ammonium at these pH levels.
Areas of Agreement / Disagreement
Participants express differing views on the explanations for the stability of aluminum and the effects of pH on the reaction, indicating that the discussion remains unresolved with multiple competing perspectives.
Contextual Notes
There are limitations in the assumptions made regarding the behavior of aluminum in various pH environments, and the discussion reflects a lack of consensus on the underlying mechanisms at play.