- #1
lucas_
- 413
- 23
Molecules don't really look like this with clearly defined objects and outlines. Remember that in the double slit experiments, we can't even model what happens between measurements, like how the electron behave between emitter and detector.
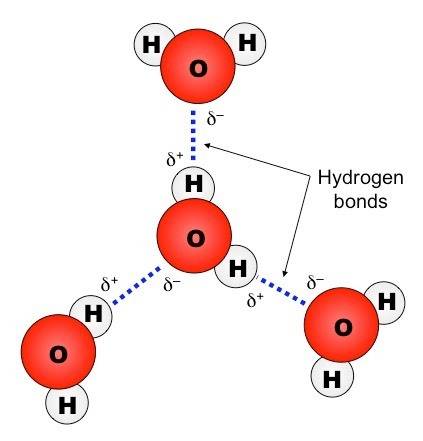
So what is the best model to describe them? How can molecules interact using without the concept of wave function or hamiltonians? How do you visualize models physically?
Are there more exotic ways that they can interact? What's the latest in Atomic and Condensed Matter study about this all?
So what is the best model to describe them? How can molecules interact using without the concept of wave function or hamiltonians? How do you visualize models physically?
Are there more exotic ways that they can interact? What's the latest in Atomic and Condensed Matter study about this all?