pisluca99
- 63
- 4
Generally, a bathochromic shift is observed when there is conjugation between more chromophore groups, that is, an increase in the absorption lambda max is observed. But what lambda max is being referred to?
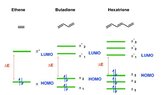
For example, comparing ethene and butadiene there is a batochromic shift only if we consider the π2 --> π*1 transition (see image), that is, the HOMO-LUMO transition.
In contrast, if we go to consider the π1 --> π*2 transition of butadiene, a hypsochromic shift is observed, as the lambda max relative to this transition is smaller than the lambda max relative to the π --> π* transition of ethene.
That said, does the red shift, then, ALWAYS refer to the HOMO-LUMO transition or does it affect other orbitals?
Another example is that of ethene and vinyl chloride: in this case we add an auxochrome group (Chlorine) that increases conjugation. Because of this, we observe an increase in the absorption lambda max, as well as a red shift. Again, does the increase in lambda max always refer to the HOMO-LUMO transition or to other orbitals?
For example, comparing ethene and butadiene there is a batochromic shift only if we consider the π2 --> π*1 transition (see image), that is, the HOMO-LUMO transition.
In contrast, if we go to consider the π1 --> π*2 transition of butadiene, a hypsochromic shift is observed, as the lambda max relative to this transition is smaller than the lambda max relative to the π --> π* transition of ethene.
That said, does the red shift, then, ALWAYS refer to the HOMO-LUMO transition or does it affect other orbitals?
Another example is that of ethene and vinyl chloride: in this case we add an auxochrome group (Chlorine) that increases conjugation. Because of this, we observe an increase in the absorption lambda max, as well as a red shift. Again, does the increase in lambda max always refer to the HOMO-LUMO transition or to other orbitals?
Attachments
Last edited: