- #1
humphreybogart
- 22
- 1
The continuity equation in fluid mechanics is:
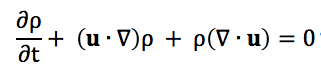
Do the condition of "constant-density fluid' and 'imcompressible flow' have the same effect on the continuity equation, in that the first two terms disappear?
Or is there a difference between these assumptions?
Do the condition of "constant-density fluid' and 'imcompressible flow' have the same effect on the continuity equation, in that the first two terms disappear?
Or is there a difference between these assumptions?