Discussion Overview
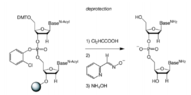
The discussion revolves around drawing the full mechanism for chemical reactions in steps 1 and 2, specifically focusing on the use of protecting groups and the role of Tetramethylguanidine (TMG) and monomethoxytrityl (MMT). Participants are seeking clarification and guidance on how to accurately depict these mechanisms.
Discussion Character
- Homework-related
- Technical explanation
- Conceptual clarification
Main Points Raised
- One participant requests help in drawing the full mechanism for the reactions in steps 1 and 2, indicating specific reagents and protecting groups involved.
- Another participant suggests that the original poster should show some work and identify which reagent deprotects which functional group to facilitate assistance.
- The original poster expresses uncertainty about their mechanism and shares their approach, seeking validation.
- A subsequent reply acknowledges the original poster's idea but points out minor errors regarding the representation of protecting groups in the mechanism diagrams.
Areas of Agreement / Disagreement
The discussion does not reach a consensus, as participants provide feedback and corrections without establishing a definitive solution to the mechanism drawing task.
Contextual Notes
Participants note specific errors related to the representation of protecting groups, indicating that attention to detail is necessary in the mechanism drawings. There is also an acknowledgment of uncertainty regarding the correctness of the original poster's approach.
Who May Find This Useful
Students or individuals working on organic chemistry mechanisms, particularly those involving protecting groups and reaction steps.