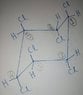
SUMMARY
The discussion centers on the number of halogens removed in E2 reactions, specifically addressing the role of chlorines and the necessity for anti-periplanar geometry. Participants analyze the implications of using PBr3 for substitution and its potential to lead to elimination reactions. The consensus indicates that E2 mechanisms require specific spatial arrangements of atoms, and that the reactions may yield complex mixtures of products. The dialogue emphasizes the importance of understanding stereochemistry and the conditions under which elimination occurs.
PREREQUISITES
- Understanding of E2 elimination mechanisms
- Familiarity with stereochemistry, specifically anti-periplanar and synperiplanar arrangements
- Knowledge of nucleophilic substitution reactions, particularly SN2
- Experience with reagents like PBr3 and NaI in organic reactions
NEXT STEPS
- Research the specifics of E2 elimination mechanisms and their stereochemical requirements
- Study the effects of different solvents on nucleophilic substitution reactions, particularly polar aprotic solvents
- Explore the Finkelstein reaction and its implications for substitution and elimination
- Investigate the role of leaving groups in E2 reactions and how they influence product formation
USEFUL FOR
Chemistry students, organic chemists, and anyone interested in understanding elimination reactions and stereochemical principles in organic synthesis.