r12214001
- 24
- 2
- Homework Statement
- HF molecular orbital
- Relevant Equations
- HF molecular orbital
In figure. Why F use 2P rather than 2S to bonding in HF MO model.
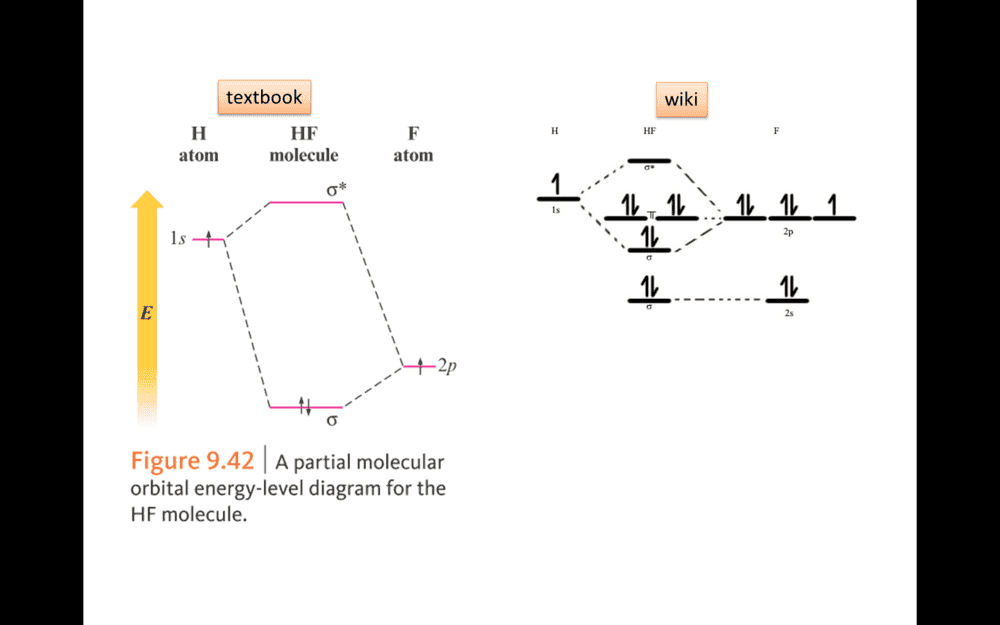
The discussion centers on the bonding characteristics of fluorine (F) in the context of the HF molecular orbital (MO) model. Fluorine has an electron configuration of 1s2 2s2 2p5, indicating that it utilizes its 2p orbitals for bonding rather than the filled 2s orbital. Participants clarify that hybridization should not be used to explain MO theory, yet acknowledge that fluorine can exhibit sp3 hybridization when forming bonds. The bonding electrons in fluorine primarily reside in the 2p orbital.
PREREQUISITESChemistry students, molecular modelers, and educators seeking to deepen their understanding of bonding in molecular orbital theory, particularly in relation to fluorine and hybridization concepts.
1S2 2S2 2P5Borek said:What is the electron configuration of a free F atom?
OK i get it. I should never use hybridization to account for MO theory.chemisttree said:Can you bond using suborbitals that are already filled?